Last week Mike the Mad Biologist linked to an essay by Union College physics professor Chad Orzel, “Particle And Astro Aren’t The Only Kinds Of Physics“. Orzel’s field is Atomic, Molecular, and Optical (AMO) Physics. For my Ph.D. research I worked a problem in molecular physics. It was an experimental test of a very old theoretical problem: Imagine a hydrogen atom with a huge point electric dipole moment at its nucleus. What happens to the hydrogenic orbitals and the energy levels as a result of the dipole? That was one aspect of the problem. The other was that there is no such thing as an atomic ion with an huge electric dipole moment at its nucleus. The Rydberg states I was studying had a molecular ion core rather than an atomic one. The other question I addressed was how an the oscillating dipole affects the exchange of energy and angular momentum between the Ryberg electron and the molecular ion core. Anyhow, with our backgrounds in AMO physics, Orzel and I share some common experience and common knowledge. His article resonated with me. He starts out:
I sometimes get asked why I’m not a particle physicist. This is a question that can have a bit of an edge, particularly when coming from high-energy theorists, a number of whom feel that only second-rate physicists do experimental particle physics, and anyone who studies things larger than the nucleus of an atom might as well be a chemist.
I sympathize. My lab was the Chemistry Dept. I’d often get asked why I wasn’t an organic chemist. Orzel provides a really nice explanation for why he chose the field he did:
I’m in AMO physics by choice, because I find it a particularly congenial field for a number of reasons, some of which I’ll try to explain here… It’s not too big… [In contrast to particle physics, the] community as a whole is also much smaller… This means you have an excellent chance of getting to see and interact with even the biggest names in the field, who are mostly very down-to-Earth folks… It’s not too small… The subject matter of AMO physics, as it says right there in the name, involves atoms and molecules, generally very simple molecules by the standards of chemistry. These hit a sort of Goldilocks point, at least for me– they’re small enough to show interesting quantum effects, but not so small that you can’t see them directly… The physics is amazing… It has applications all over… You can even attack the same fundamental physics studied with giant particle colliders using small-scale AMO physics labs…. (As a bonus, [AMO is] also an unusually collegial subfield– even research groups that are in direct competition tend to be on friendly terms with one another…)
It was a nice essay and also timely in that we held a mini-symposium for my Ph.D. advisor last weekend in honor of his 71st birthday. (Why 71? Because we, his former grad students, didn’t get organized in time to do it for his 70th.) It was great to catch up with people I hadn’t seen in 10-20 years.
The guest speakers gave some very good talks. I enjoyed them all. For the majority of them though, while I understoodThe Big Picture, I’ve been away from the field for long enough that the details went over my head. To be expected I suppose. For two of the talks though, it was like hearing a language in which I was once fluent but hadn’t spoken in 15 years. There were gaps in understanding initially but details came back to me as the presentations progressed. One of those talks was by Steve Leone. He spoke about photoionization of Ne and other rare gases using attosecond-scale laser pulses. I won’t attempt to describe the details but here’s the Abstract to a recent article from his group (emphasis mine):
Electronic wavepackets composed of multiple bound excited states of atomic neon lying between 19.6 and 21.5 eV are launched using an isolated attosecond pulse. Individual quantum beats of the wavepacket are detected by perturbing the induced polarization of the medium with a time-delayed few-femtosecond near-infrared (NIR) pulse via coupling the individual states to multiple neighboring levels. All of the initially excited states are monitored simultaneously in the attosecond transient absorption spectrum, revealing Lorentzian to Fano lineshape spectral changes as well as quantum beats. The most prominent beating of the several that were observed was in the spin–orbit split 3d absorption features, which has a 40 femtosecond period that corresponds to the spin–orbit splitting of 0.1 eV. The few-level models and multilevel calculations confirm that the observed magnitude of oscillation depends strongly on the spectral bandwidth and tuning of the NIR pulse and on the location of possible coupling states.
Leone also presented evidence that in Ne the 2p electron ionizes ~20 attoseconds faster than the 2s. If you’re not an AMO physicist then you’re reaction is probably, “Umm… That’s nice.” but if you are one then it’s great stuff. (And, if you haven’t already done the math, an attosecond is a billionth of a billionth of second. Light only travels the length of a few dozen chemical bonds over the duration of laser pulses they were using. That’s a very short distance.) If you’re interested here’s a pdf of one of the Leone group’s recent publications:
- X. Li, et al., “Investigation of coupling mechanisms inattosecond transient absorption of autoionizing states: comparison of theory and experiment in xenon“, J. Phys. B: At. Mol. Opt. Phys., vol. 48 (2015), paper no. 125601.
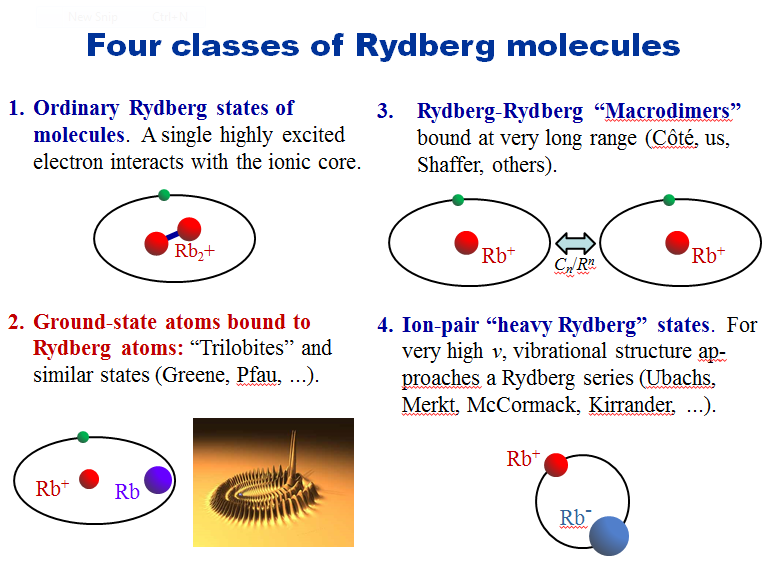
The talk that I enjoyed most however was by UConn Prof. Ed Eyler. He spoke about “Rydberg molecules”. Ed is working on somewhat different problems than I did but but there’s sufficient overlap that I appreciate the details. I was tickled that work I’d done 20+ years ago featured in one of his slides but, more than that, I was taken by what’s happened in the field since I finished by dissertation. There was one chart in particular which caught my attention:
Ordinary Rydberg molecules have been known for ages – close to a century now? Greene and coworkers predicted “trilobite-like” polar homonuclear diatomics in 2000 but Classes 2 thru 4 were discovered more recently. The trilobite-like molecules exhibit a new kind of chemical bond – not ionic, not covalent, a new kind of chemical bond. There’s also
- Ultralong-range Ryberg molecules
- A Homonuclear Diatomic Permanent Electric Dipole Moment and
- Observation of trilobite-like Rydberg states
Ed also speculated that Rydberg crystals may not be far off. Fascinating stuff.
Great talks and a great get together. It’s times like those where I really miss being a researcher. Orzel was spot on with his observation about the field: “You have an excellent chance of getting to see and interact with even the biggest names in the field, who are mostly very down-to-Earth folks.”
The symposium attendees: